The Kopp lab utilizes multidisciplinary and innovative approaches to advance our understanding as to how innate immunity contributes to the pathogenesis of chronic lung diseases. We focus on host-pathogen interactions that impact chronic respiratory diseases such as cystic fibrosis (CF) and lung disease related to sickle cell. The two main areas of this research include how innate immune cells fail to regulate infection in chronic lung diseases as well as elucidating biomarkers/pathways of early inflammatory airway disease. We use the information gained to help develop novel therapeutics and targets to allow for improved killing of bacteria and regulation of hyper-inflammatory signaling in chronic respiratory diseases. Our passion and vision are to rapidly translate research discoveries into effective treatment and prevention strategies for children and adults afflicted with chronic respiratory diseases such as CF and sickle cell. To achieve these goals, we actively engage in translating our findings from the bench to the clinic whenever possible, aligned with Dr. Kopp’s goals as a physician-scientist.
Contributions to Science:
1) Fundamental defects in macrophage function dependent on CFTR. Why bacterial infections in the CF airway are established so early in life remains poorly understood. We recently discovered that CF macrophages have a defective assembly of the NADPH oxidase, which leads to reduced reactive oxygen species (ROS) production, and therefore reduced bacterial killing. The defective assembly is characterized by reduced calcium-dependent protein-kinase-C-mediated phosphorylation of cytosolic NADPH components.a As ROS production is one of the earliest responses to infection, we have determined a crucial initiating step for the early establishment of intracellular survival in CF macrophages. This critical finding is a focus of ongoing studies in the CF host immune response to bacterial pathogens. As part of a large project to determine how loss of CFTR influences macrophage function, we developed a CRISPR-Cas9 CFTR human macrophage model in which we verified that defective NADPH oxidase assembly is directly dependent on CFTR.b We also helped identify similar deficits in CF neutrophil ROS production.c Finally, we identified mechanisms by which CFTR modulation partially restores macrophage function and immunophenotypic markers of clinical responses.d
a. Assani K, Shrestha CL, Robledo-Avila F, Rajaram MV, Partida-Sanchez S, Schlesinger LS, Kopp BT. “Human Cystic Fibrosis Macrophages Have Defective Calcium-Dependent Protein Kinase C Activation of the NADPH Oxidase, an Effect Augmented by Burkholderia cenocepacia”. J Immunol. 2017 Jan 16. PMID: 28093527.
b. Zhang, S, Shrestha CL, Wisniewski B, Pham H, Hou X, Li W, Dong Y, Kopp BT. “Consequences of CRISPR-Cas9-mediated CFTR knockout in human macrophages.” Frontiers in Immunology. 2020 July. PMID: 32973772.
c. Robledo-Avila FH, Ruiz-Rosado JD, Brockman KL, Kopp BT, Amer AO, McCoy K, Bakaletz LO, Partida-Sanchez S. Dysregulated Calcium Homeostasis in Cystic Fibrosis Neutrophils Leads to Deficient Antimicrobial Responses. J Immunol. 2018 Oct 1;201(7):2016-2027. PMID: 30120123.
d. Zhang S, Shrestha C, Robledo F, Jaganathan D, Wisniewski B, Brown N, Pham H, Carey K, Amer A, Hall-Stoodley L, McCoy K, Bai S, Partida-Sanchez S, Kopp BT. Cystic fibrosis macrophage function and clinical outcomes after elexacaftor/tezacaftor/ivacaftor. European Respiratory Journal, 2022.
2) Critical pathways of CF macrophage-mediated bacterial killing that respond to therapeutics. We have determined that human CF macrophages have reduced autophagy, which leads to impaired macrophage-mediated bacterial killing. Reduced macrophage autophagy is dependent on transglutaminase 2 (TG2)-mediated sequestration of autophagy initiating molecules, which is reversed by administration of the TG2 inhibitor cysteamine.a Autophagy and CFTR can be independently stimulated through our novel agent, AR-13, which results in clearance of multi-drug resistant bacteria from primary immune cells (U.S. Patent application 10028933).b Further, macrophage responses to CFTR modulation reveals additional targets for therapeutic manipulation of host defenses.c Using RNA-Seq we have also determined novel therapeutic pathways induced by CFTR modulators that can be targeted for future immunomodulation in CF.d
a. Shrestha CL, Assani KD, Rinehardt H, Albastroiu F, Zhang S, Shell R, Amer AO, Schlesinger LS, Kopp BT. Cysteamine-mediated clearance of antibiotic-resistant pathogens in human cystic fibrosis macrophages. PLoS One. 2017 Oct 5;12(10). PMID: 28982193.
b. Assani K, Shrestha CL, Rinehardt H, Zhang S, Robledo-Avila F, Wellmerling J, Partida-Sanchez S, Cormet-Boyaka E, Reynolds SD, Schlesinger LS, Kopp BT. AR-13 reduces antibiotic-resistant bacterial burden in cystic fibrosis phagocytes and improves CFTR function. J Cyst Fibros. 2018 Oct 23. PMID: 30366849.
c.Zhang S, Shrestha CL, Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep. 2018 Nov 20;8(1):17066. PMID: 30459435.
d.Kopp BT, Fitch J, Jaramillo L, Shrestha CL, Robledo-Avila F, Zhang S, Palacios S, Woodley F, Hayes D Jr, Partida-Sanchez S, Ramilo O, White P, Mejias A. Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J Cyst Fibros. 2019 Aug 29; PMID: 31474496.
3) Characterized factors determining Burkholderia cenocepacia survival in human CF macrophages. To model bacterial persistence in CF and the efficacy of potential therapeutics, I have studied the persistence of B. cenocepacia in human CF macrophages. B. cenocepacia is highly virulent in patients with CF, underscoring its importance in modeling. We first determined that B. cenocepacia uses an O antigen to stimulate caspase-1 dependent IL-1β production and resulting host damage.a We then determined that human macrophages respond to B. cenocepacia with an exaggerated inflammatory cytokine response including IL-1β.b Recently, we discovered that bacterial intracellular survival occurs via reduced autophagy and therefore results in excess cytokine production. Reduced autophagy can be reversed with autophagy stimulating agents such as IFN-yc and is controlled by a microRNA cluster that also negatively regulates CFTR and is predictive of clinical outcomes.d
a. Kotrange S, Kopp B, Akhter A, Abdelaziz D, Abu Khweek A, Caution K, Abdulrahman B, Wewers MD, McCoy K, Marsh C, Loutet SA, Ortega X, Valvano MA, Amer AO. Burkholderia cenocepacia polysaccharide chain contributes to caspase-1-dependent IL-1beta production in macrophages. J Leukoc Biol. 2011 Mar;89(3):481-8. PMID: 21178113; PMCID: PMC3040464.
b. Kopp BT, Abdulrahman BA, Khweek AA, Kumar SB, Akhter A, Montione R, Tazi MF, Caution K, McCoy K, Amer AO.” Exaggerated inflammatory responses mediated by Burkholderia cenocepacia in human macrophages derived from Cystic fibrosis patients.” Biochem Biophys Res Commun. 2012 Jul 27;424(2):221-7. PMID: 22728038; PMCID: PMC3408781.
c. Assani K, Tazi MF, Amer AO, Kopp BT. “IFN-γ stimulates autophagy-mediated clearance of Burkholderia cenocepacia in human cystic fibrosis macrophages.” PLoS One. 2014 May 5;9(5):e96681. PMID: 24798083.
d. Krause K*, Kopp BT*, Tazi MF, Caution K, Hamilton K, Badr A, Shrestha C, Tumin D, Hayes D Jr, Robledo-Avila F, Hall-Stoodley L, Klamer BG, Zhang X, Partida-Sanchez S, Parinandi NL, Kirkby SE, Dakhlallah D, McCoy KS, Cormet- Boyaka E, Amer AO. The expression of Mirc1/Mir17-92 cluster in sputum samples correlates with pulmonary exacerbations in cystic fibrosis patients. J Cyst Fibros. 2018 Jul;17(4):454-461. PMID: 29241629.
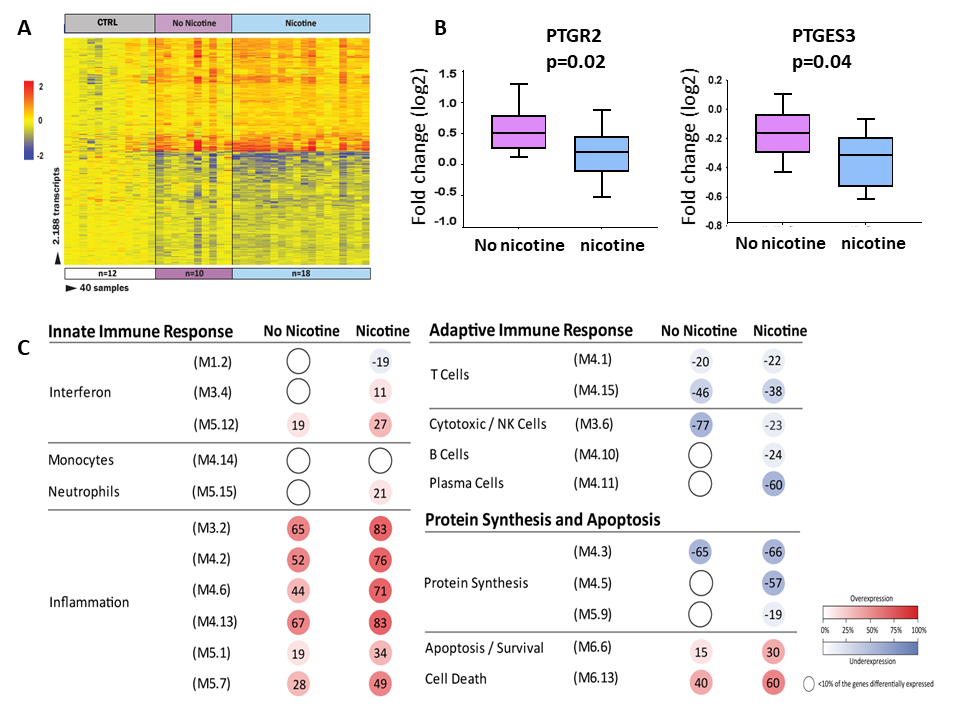
4) The impact of secondhand smoke exposure on early disease progression and survival in children with CF. Concurrent with our studies on host immune function, I am interested in how environmental modifiers can modulate immunity in CF. I was the first investigator to identify second-hand smoke as a risk factor for poor growth and bacterial acquisition in infants with CF.a Currently we are prospectively examining the correlation between nicotine levels in infants and inflammatory gene expression and immune cell function in CF. We have discovered that secondhand smoke exposure alters arachidonic acid metabolism, which is associated with heightened inflammation and increased bacterial burden.b Further, we have identified alterations in the intestinal microbiome that are correlated with smoke exposure in young children.c Finally, we identified critical metabolic pathways altered by tobacco exposure in the same cohort.d
a. Kopp BT, Sarzynski L, Khalfoun S, Hayes D Jr, Thompson R, Nicholson L, Long F, Castile R, Groner J. Detrimental effects of secondhand smoke exposure on infants with cystic fibrosis. Pediatr Pulmonol. 2014 Mar 9. PMID: 24610820.
b. Kopp BT, Thompson R, Kim J, Konstan R, Diaz A, Smith B, Shrestha C, Rogers LK, Hayes D, Tumin D, Woodley F, Ramilo O, Sanders DB, Groner J, Mejias A. Secondhand smoke alters arachidonic acid metabolism in infants and children with cystic fibrosis. Thorax. Jan 19, 2019. PMID: 30661024.
c. Loman BR, Shrestha CL, Thompson R, Groner JA, Mejias A, Ruoff KL, O'Toole GA, Bailey MT, Kopp BT. Age and environmental exposures influence the fecal bacteriome of young children with cystic fibrosis. Pediatr Pulmonol. 2020 Apr 10. PMID: 32275127.
d. Wisniewski B, Shrestha CL, Zhang S, Thompson R, Gross M, Groner J, Uppal K, Mejias M, Kopp BT. Metabolomics profiling of tobacco exposure in children with cystic fibrosis. J Cyst Fibros. 2020 May. PMID: 32487493.
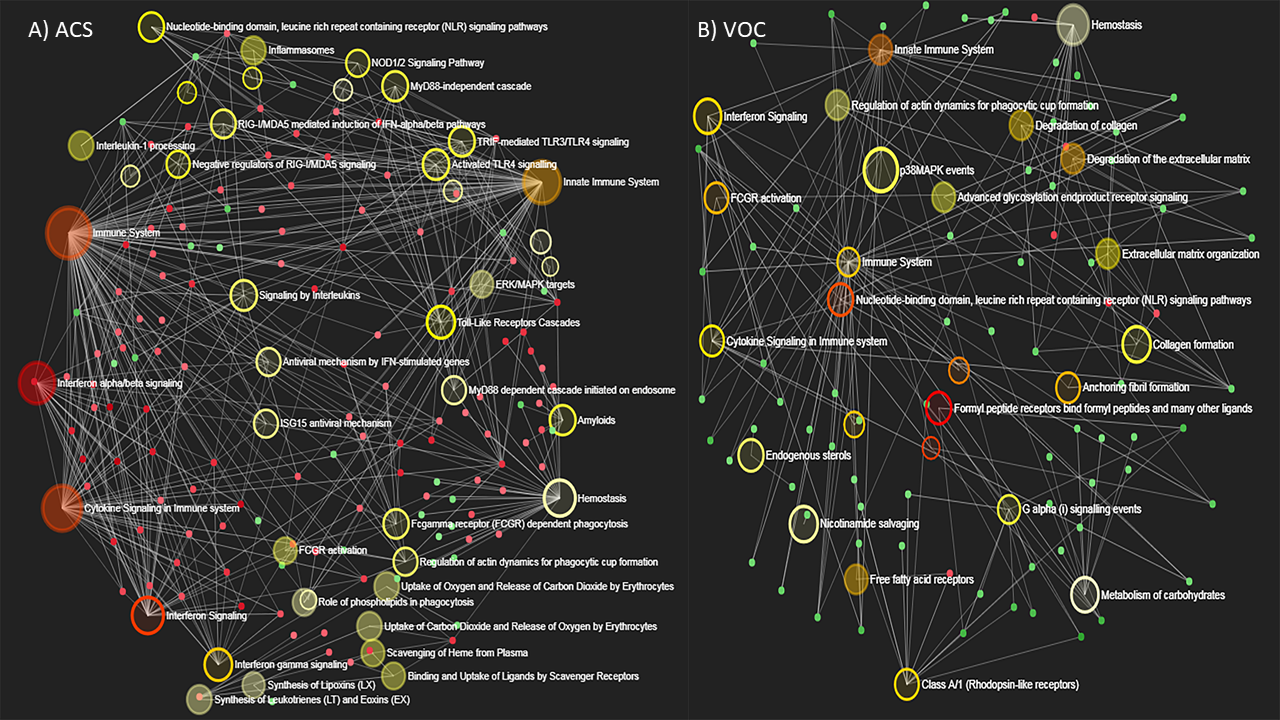
5) Discovered novel alterations in gene regulatory networks and microbial interactions during acute chest syndrome in children with sickle cell disease (SCD). We are currently using a multi-omics approach to better understand acute and chronic lung disease in children with SCD. We are the first to publish alterations in the upper airway microbiome of children with SCD at baseline, during acute chest syndrome, and during vaso-occlusive episodes.a We are also the first to describe whole-blood RNA-seq transcriptional profiles in this same cohort.b Overall, these findings will continue to help drive the research recommendations for lung disease in children with SCD.c,d
a. Creary S, Loman BR, Kotha K, Shrestha CL, Minta A, Zhang S, Pinto S, Thompson R, Mejias A, Bailey MT, and Kopp BT. Upper airway microbiome changes in children with sickle cell during acute chest syndrome. Am J Hematology. 2020 July 9. PMID: 32644239.
b. Creary S, Shrestha CL, Kotha K, Minta A, Fitch J, Jaramillo L, Zhang S, Pinto S, Thompson R, Ramilo O, White P, Mejias A, Kopp BT. Baseline and disease-induced transcriptional profiles in children with sickle cell disease. Sci Reports (Nature). 2020 Jun. PMID: 32487996.
c. Ruhl AP, Sadreameli SC, Allen JL, Bennett DP, Campbell AD, Coates TD, Diallo DA, Field JJ, Fiorino EK, Gladwin MT, Glassberg JA, Gordeuk VR, Graham LM, Greenough A, Howard J, Kato GJ, Knight-Madden J, Kopp BT, Koumbourlis AC, Lanzkron SM, Liem RI, Machado RF, Mehari A, Morris CR, Ogunlesi FO, Rosen CL, Smith-Whitley K, Tauber D, Terry N, Thein SL, Vichinsky E, Weir NA, Cohen RT, Klings ES. Identifying Clinical and Research Priorities in Sickle Cell Lung Disease. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2019 Sep. PMID: 31469310.
d. Sadreameli SC, Kopp BT, Creary SE, Eakin MN, McGrath-Morrow S, Strouse JJ. Secondhand Smoke Is an Important Modifiable Risk Factor in Sickle Cell Disease: A Review of the Current Literature and Areas for Future Research. Int J Environ Res Public Health. 2016 Nov 12;13(11):1131. PMID: 27845754.
Postdoctoral Fellows
Ben Wisniewski, MD, 2018 – 2021, Currently Assistant Professor of Pediatrics, Colorado Children’s
Devi Jaganathan PhD, 2022, Currently Post-doctoral fellow Nationwide Children's
Laboratory Staff scientists
Shuzhong Zhang, PhD, 2016-2021
Chandra Shrestha, PhD, 2015- 2022
Katherine Carey, 2019-2022
Kaivon Assani, 2012-2016
Graduate Students
Amelia Cephas, 2018
Pediatric Residents
Lisa Ulrich MD, 2012, Currently Assistant Professor of Pediatrics, Nationwide Children’s Hospital
Sabrina Palacios MD, 2013, Currently Assistant Professor of Pediatrics, Nationwide Children’s Hospital
Medical/Undergraduate/High School Interns
Avish Jain, Undergraduate intern, Ohio State University, 2014 – 2016
Hannah Rinehardt, Undergraduate intern, Ohio State University, 2014 – 2016
Florentina Albastroiu, Undergraduate intern, Ohio State University, 2014 – 2016
Jeeho Kim, Roessler Medical Student Research Scholar, Ohio State University, 2015 – 2018
Abena Minta, Undergraduate intern, Ohio State University, 2016 – 2018
Abdul Bah, Undergraduate intern, Ohio State University, 2016 – 2018
Robert Konstan, Undergraduate intern, Ohio State University, 2016 – 2018
Kristina Myers, Summer intern, Ohio Northern, 2018
Emily McCauley, Undergraduate intern, Ohio State University, 2018 – 2020
Nevian Brown, Undergraduate intern, Ohio State University, 2019 – 2021
Courtney Pugh, Undergraduate intern, Ohio State University, 2020 – 2022
Ashely Thomas, Undergraduate intern, Ohio State University, 2022 – present
Dinesh Bojja, High School intern, Olentangy Schools, 2022
Full listing of publications at:
Pubmed Page
Google Scholars Page