Overview
The Integrated Cellular Imaging Core (ICI) provides access to cutting-edge cellular imaging technologies and technical expertise to support pediatric research
ICI is a partnership facilitated by the Emory School of Medicine and Winship Cancer Institute. ICI incorporates the Pediatric Research Alliance Cellular Imaging Core along with other cellular imaging sites on campus including Winship, Emory NINDS Neuroscience Core Facilities (ENNCF) and the Department of Physiology. The scientific direction of ICI is led by Adam Marcus, PhD and the Health Sciences Research Building (HSRB) site is managed by Neil Anthony, PhD. Their efforts will help ensure all cellular microscopy research at Emory generate maximum quality data with the highest scientific impact possible. Additionally, ICI organizes educational events and seminars to facilitate scientific exchanges. Please contact them at ici@emory.edu if you would like to be included on the ICI listserv to learn about these events and to receive other information relevant to cellular microscopy.
The rates for all the microscopes included in this effort are located on the pricing page. Please click here for details on pricing and subsidy.
ICI is one of the Emory Intergrated Core Facilities (EICF), to learn more about EICF and other core services please see here.
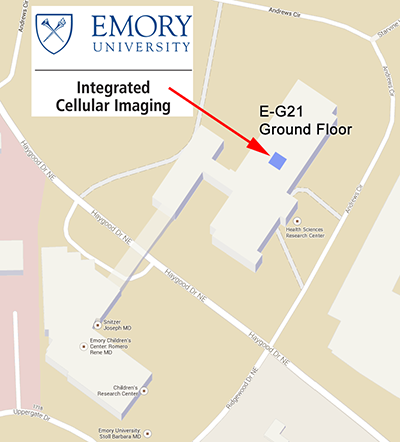
The pediatric component of the Integrated Cellular Imaging Core is located in the Emory Health Sciences Research Building (HSRB) on the ground floor, E-G21.
Bookings can be made online for the scopes through PPMS. To view the calendars, please click here. Pediatric users are also welcome to use microscopes at any of the ICI locations.
For further assistance regarding this core, please contact ici@emory.edu
Olympus FV1000 Confocal Laser Scanning Microscope (CLSM)
The Fluoview hardware allows for fluorescence imaging using 6 different laser lines and advanced spectral filters. This facilitates imaging of nearly all available fluorescent dyes and proteins. Confocal microscopy provides optical sectioning, providing enhanced contrast compared to standard widefield microscopes, and allowing for detailed 3D data acquisition.
Commonly used functions:
- Multi-color acquisitions
- Colocalization
- 3D analyses
- FRET
Olympus IX71 Inverted Microscope
Basic microscope with fluorescent filter sets and CCD camera for quick image acquisitions. Standard log on to computer and booking system for image acquisition using software controlled camera.
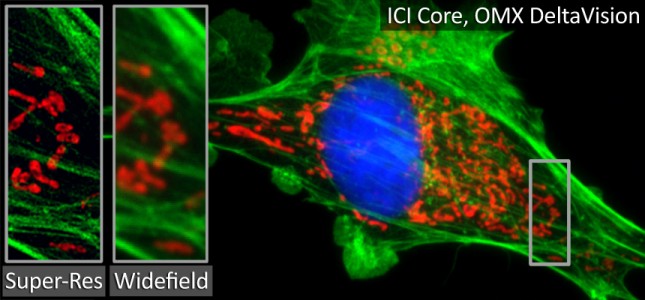
GE DeltaVision OMX Blaze
Features:
- Live cell super-resolution (heated objective and sample chamber with 5% CO2)
- 2D and 3D Structured Illumination Microscopy (SIM)
- Widefield Deconvolution
- Ring TIRF
- DIC
- Photostimulation for techniques such as FRAP, PA-FGP, uncaging, and FLIP
- Motorized stage for 4D acquisition and multiple stage points
- Laser based hardware autofocus
- 3x sCMOS cameras for ultra-fast multi-color imaging
Objectives:
- 60X 1.42 NA, Oil
- 60X 1.49 NA, Oil TIRF
Laser Lines (nm):
- 405, 488, 568, 642
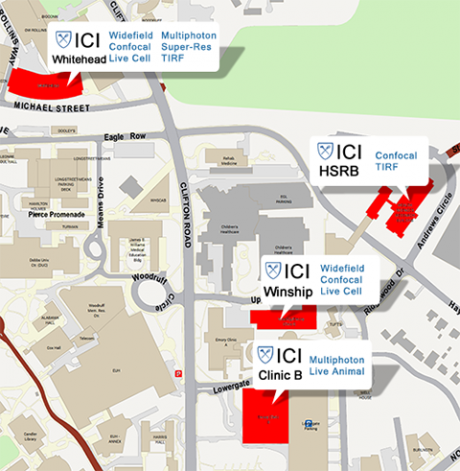
ICI provides state-of-the-art microscopes and support across four satellite locations on campus. For additional details, please click here.
A wide range of microscope are available, allowing researchers an almost endless range of imaging options. Please let us know if you have any questions about what microscopes and techniques would be best for your experiments, email ici@emory.edu
Types of Imaging:
- Confocal
- Live Cell
- Widefield
- Multiphoton
- Super Resolution
- Small Animal Imaging System
- Roche xCELLigence Plate Reader
Please click here to view the ICI rates.
The Integrated Cellular Imaging Core is generously supported by Children's Healthcare of Atlanta and Emory University. When presenting or publishing work completed using the core, please include "Children's Healthcare of Atlanta and Emory University's Pediatric Integrated Cellular Imaging Core" in the acknowledgments.
To determine if you should use “Children’s Healthcare of Atlanta” in your author affiliations, please see guidelines here.
"The Next Level of Fluorescence"
Awardees
Edwin Horwitz: Fluorescence Microscopy Characterization of γMSC phagocytosis
Rabindra Tirouvanziam: Imaging PD1 and Extracellular Vesicle Signaling in Cystic Fibrosis
Curtis Henry: Adipocyte-secreted factors affect chronic inflammation in Acutelymphoblastic leukemia
Crystal Naudin (Rheinallt Jones): 3-dimensional fibrotic scar reconstruction by immunofluorescent microscopy of whole-mounted mouse left ventricular tissue
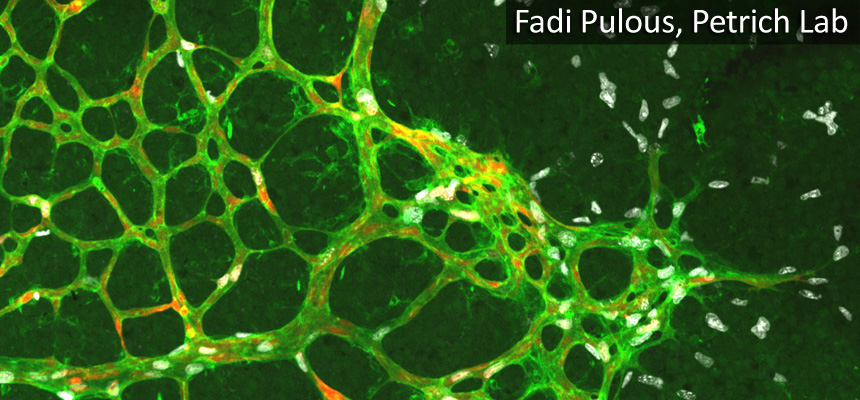
Brian Petrich: Localization pattern of b1 integrin and talin at endothelial cell-cell junction

