Please visit our new lab website for the most up to date information!!
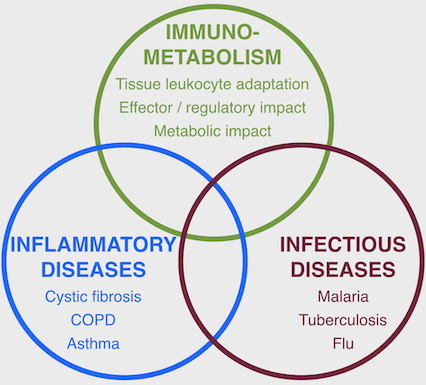
The Tirouvanziam Lab @ Emory uses deep phenotyping methods to investigate mechanisms of immune and metabolic dysfunction in human inflammatory and infectious diseases
Please visit our new lab website for the most up to date information!!
History:
Previously at the Institute of Embryology at CNRS / College de France in Paris, France (1994-99), and at Stanford University in California (1999-2011), Dr. Tirouvanziam has led efforts to revisit the biology of innate immune subsets in human, mouse, SCID-Hu xenografts, and Drosophila.
Focus:
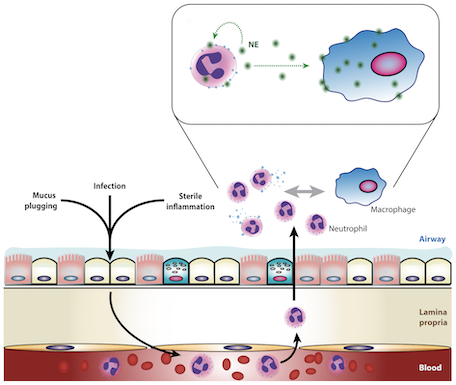
Research in the Tirouvanziam Lab @ Emory includes patient- and model-based studies of lung diseases such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), flu, asthma, as well as other disease conditions, such as food allergy, malaria, tuberculosis, and cancer. Active areas of research in the laboratory include: (i) epigenetic and transcriptional reprogramming in tissue-recruited leukocytes; (ii) extracellular vesicle-mediated crosstalk in tissue immunity; (iii) predictive biomarkers of early CF airway disease; (iv) drug development for intractable human conditions leveraging new knowledge on tissue-recruited leukocytes.
Approach:
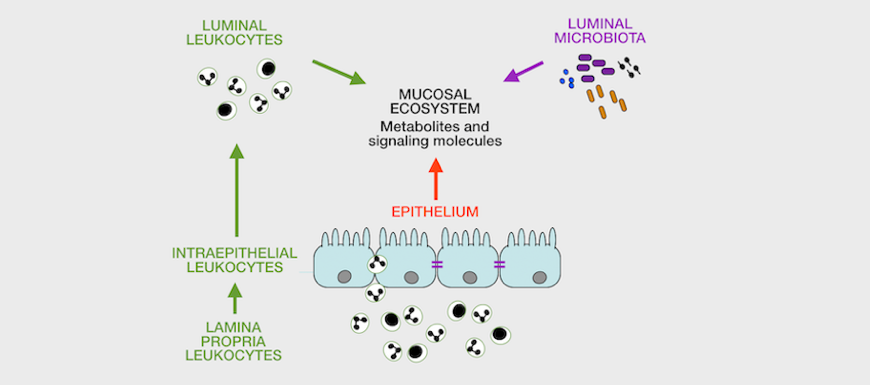
The laboratory emphasizes sample analysis from patients and animal models using direct, high-content analyses by flow / image cytometry / transcriptomics / proteomics (for cells), proteomics and metabolomics (for fluids) to study immunometabolic pathways and their relation to disease in vivo. We also develop custom, feature-rich in vitro systems for drug screening / testing. Finally, we are interested in identifying bettertargets for therapy and in identifying endpointsto improve immunometabolic monitoring at the clinic and at home.
Funding:
Current funding sources include NIH (R01), the US CF Foundation (basic research and clinical trial awards), DARPA (THoR and PREPARE programs), as well as industry contracts.
Collaborations:
Our laboratory has a strong ecosystem of partners. Near: Emory and Georgia Tech in Atlanta; University of Georgia in Athens; University of Alabama in Birmingham. Far: Erasmus University in Rotterdam, The Netherlands; Telethon Kids Institute in Perth, Australia; Charité Hospital in Berlin, Germany; University of Reims, France.
Contributions to science
1. Development of a xenograft model of human lung development: My work in the field of lung biology and CF started 20 years ago, with the development and experimental use of a xenograft model of human lung development. I was the primary scientist in charge of developing this model as a graduate student at CNRS-College de France in Paris [Refs. a, b] and continued to use it as a postdoctoral fellow at Stanford University in collaborative studies with my graduate laboratory [Refs. c, d]. This xenograft model offered a unique window into the ontogeny of airway mucosal immune structures [Ref. c], and human CF disease prior to any infection. Unexpectedly, we observed a primary defect in the regulation of neutrophilic inflammation in CF [Ref. d]. This seminal result pushed me to investigate this defect in patients during my postdoctoral training (see below).
a. Tirouvanziam R, ..., Chinet T. Bioelectric properties of human CF and non-CF fetal tracheal xenografts in SCID mice. Am J Physiol. 1998; 274:C875-82. PMID: 9575783
b. Tirouvanziam R, ..., Puchelle E. Inflammation and infection in naive human CF airway grafts. Am J Respir Cell Mol Biol. 2000; 23(2):121-7. PMID: 10919974
c. Tirouvanziam R, ..., Péault B. Primary inflammation in human CF small airways. Am J Physiol Lung Cell Mol Physiol. 2002;283:L445-51. PMID: 12114207
d. Tirouvanziam R, ..., Péault B. Ex vivo development of functional human lymph node and bronchus-associated lymphoid tissue. Blood. 2002; 99:2483-9. PMID: 11895783
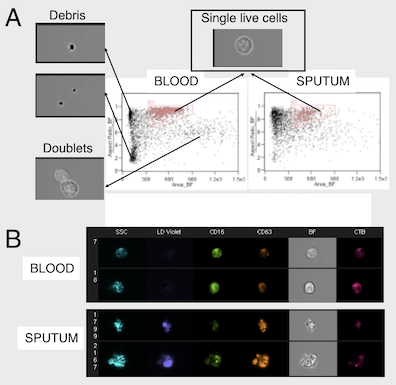
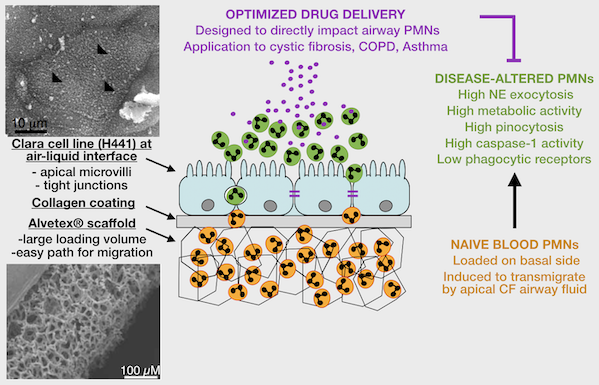
2. Patient-based studies of neutrophilic inflammation in cystic fibrosis: Based on results in the SCID-Hu model (see above), I set out as a research fellow at Stanford to revisit the process of CF airway inflammation. To this end, I developed an approach for analysis of airway samples using high-content flow / image cytometry. This led me to show that a large fraction of CF airway neutrophils is alive and releases toxic effectors actively, driving lung destruction [Refs a, b]. This finding changes the paradigm of CF airway inflammation and opens key research avenues. Recent efforts yielded evidence for reprogramming of CF airway neutrophils [Refs c, d], which we can recapitulate in vitro in a transmigration model developed in my laboratory at Emory [Ref e].
a. Tirouvanziam R, ..., Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to CF airways. PNAS. 2008; 105:4335-9. PMCID: PMC2393742. PMID: 18334635
b. Makam M, ..., Tirouvanziam R. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into CF lungs. PNAS. 2009; 106:5779-83. PMCID: PMC2667067. PMID: 19293384
c. Laval J, ..., Tirouvanziam R. Metabolic adaptation of neutrophils in CF airways involves distinct shifts in nutrient transporter expression. J Immunol. 2013; 190:6043-50. PMID: 23690474
d. Ingersoll SA, ..., Tirouvanziam R. Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death-ligand 1. J Immunol. 2015; 194:5520-8. PMCID: PMC4433848. PMID: 25926674
e. Forrest OA, ..., Tirouvanziam R. Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J Leukoc Biol. 2018; 104:665-75. PMID: 29741792
3. Drug trials and biomarker studies in CF: Using the approach for rapid ex vivoprofiling of patient samples mentioned above, I discovered a basic defect in redox regulation of CF blood neutrophils, culminating in a phase 2b clinical trial for a redox intervention [Ref. a]. These studies use optimized procedures for airway sample collection and analysis that my group leverages for all our patient-based studies, including a study of the CFTR corrector ivacaftor [Ref. b]. We also expanded our studies to include fluid-based biomarkers of CF lung disease [Ref. c], now enabling multiscale studies in CF infants [Refs. d, e].
a. Conrad C, ..., Tirouvanziam R. Long-term treatment with oral NAC: affects lung function but not sputum inflammation in CF. A phase II randomized placebo-controlled trial. J Cyst Fibros. 2015; 14:219-27. PMID: 25228446
b. Bratcher PE, ..., Tirouvanziam R*, Gaggar A*. Alterations in blood leukocytes of G551D-bearing CF patients undergoing treatment with ivacaftor. J Cyst Fibros. 2015; 15(1):67-73. PMCID: PMC456751. PMID: 25769931. *co-senior authors
c. Forrest OA, ..., Tirouvanziam R. Resistin is elevated in cystic fibrosis sputum and correlates negatively with lung function. J Cyst Fibros. 2018; in press. PMID: 29937317
d. Chandler JC, ..., Tirouvanziam R*$, Jones DP*, Janssens HM*. Myeloperoxidase oxidation of methionine associates with early cystic fibrosis lung disease. Eur Respir J. 2018; In press. PMID: 30190273. *co-senior authors, $corresponding author
e. Margaroli C, ..., Tirouvanziam R. Elastase exocytosis by airway neutrophils associates with lung damage in cystic fibrosis children. Am J Respir Crit Care Med. 2018; in press. PMID: 30281324
4. Clinical trials and ancillary studies in chronic human conditions other than CF: I contributed to several studies in allergic disease [Ref. a], chronic obstructive pulmonary disease [Ref. b], transfusion reactions [Ref. c], asthma [Ref. d], as well as autism, and malaria. These efforts have enabled me to reuse the framework and know-how developed in CF studies to study other human conditions.
a. Gernez Y, Tirouvanziam R, ..., Nadeau KC. Basophil CD203c levels are increased at baseline and can be used to monitor omalizumab treatment in nut allergy. Int Arch Allergy Immunol. 2011; 154:318-27. PMCID: PMC3214954. PMID: 2097523
b. Johnson K, ..., Tirouvanziam R, Niewoehner DE. High-dose oral N-acetylcysteine fails to improve respiratory health status in patients with COPD and chronic bronchitis: a randomized, placebo-controlled trial. Int J Chron Obstruct Pulmon Dis. 2016 Apr 21;11:799-807. PMID: 27143871
c. Fontaine MJ, ..., Tirouvanziam R. Leukocyte and plasma activation profiles in chronically transfused patients with a history of allergic reactions. Transfusion. 2017; 57(11):2639-2648. PMID: 28880378
d. Grunwell JR, Stephenson ST, Tirouvanziam R, ..., Fitzpatrick AM. Children with neutrophil-predominant severe asthma have pro-inflammatory neutrophils with enhanced survival and impaired clearance. J Allergy Clin Immunol Pract. 2018; In press. PMID: 30193935
5. Myeloid cell development / function in animal models: In parallel with translational studies, I advanced basic knowledge on effector and regulatory subsets of myeloid cells in Drosophila [Ref. a], and mice [Refs b, c, d]. This work is currently expanded in non-human primate studies, and informs our human studies.
a. Tirouvanziam R, Davidson CJ, Lipsick JS, Herzenberg LA. Fluorescence-activated cell sorting (FACS) of Drosophila hemocytes reveals important functional similarities to mammalian leukocytes. Proc Natl Acad Sci U S A. 2004; 101(9):2912-7. PMCID: PMC365719. PMID: 14976247
b. Lartey FM, Ahn GO, Shen B, Cord KT, Smith T, Chua JY, Rosenblum S, Liu H, James ML, Chernikova S, Lee SW, Pisani LJ, Tirouvanziam R, Chen JW, Palmer TD, Chin FT, Guzman R, Graves EE, Loo BW Jr. PET imaging of stroke-induced neuroinflammation in mice using Mol Imaging Biol. 2014; 16:109-17. PMCID: PMC4141125. PMID: 23836504
c. Napier RJ, Norris BA, Swimm A, Giver CR, Harris WA, Laval J, Napier BA, Patel G, Crump R, Peng Z, Bornmann W, Pulendran B, Buller RM, Weiss DS, Tirouvanziam R, Waller EK, Kalman D. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 2015; 11(3):e1004770. PMCID: PMC4379053. PMID: 25822986
d. Shin EY, Wang L, Zemskova M, Deppen J, Xu K, Strobel F, García AJ, Tirouvanziam R, Levit RD. Adenosine production by biomaterial-supported mesenchymal stromal cells reduces the innate inflammatory response in myocardial ischemia/reperfusion injury. J Am Heart Assoc. 2018;7(2). pii: e006949. PMID: 29331956
Current support
TIROUV18I0 (Tirouvanziam, Kalman) 11/01/18– 10/31/20
Cystic Fibrosis Foundation, Extramural grant
Title: Non-tuberculous mycobacteria infection in CF: a new path for therapy. The goal of this study is to assess the potential of imatinib as a repurposed host-directed therapeutic to combat difficult-to-treat infection of CF airways by non-tuberculous mycobacteria. Role: Co-PI.
APP1142505 (Garratt, Tirouvanziam, Kicic, Stick) 01/01/18 – 12/31/21
National Health and Medical Research Council of Australia, Extramural grant
Title: Epithelial drivers of neutrophil plasticity in early cystic fibrosis lung disease. This collaborative project between Telethon Kids Institute in Perth, Australia, and Emory University, Atlanta, GA, studies the impact of epithelial components of the airway fluid in early CF disease on recruited neutrophils. Role: Subcontract PI.
11702SUB (Tirouvanziam) 10/01/17 – 06/30/20
Cystic Fibrosis Foundation, Extramural contract
Title: Multicenter study of the effects of reduced glutathione on growth parameters in CF (GROW) – Laboratory assays. The GROW trial will test the impact of oral glutathione therapy on CF patients. The Tirouvanziam laboratory is contracted to run specialized laboratory assays for this trial. Role: Subcontract PI.
TIROUV17G0 (Tirouvanziam) 11/01/17– 10/31/19
Cystic Fibrosis Foundation, Extramural grant
Title: Transcriptional reprogramming of neutrophils in human CF airways. This study will use a new in vitro transmigration model and samples collected from patients to study dynamics of transcriptional reprogramming in neutrophils recruited to airways in cystic fibrosis. Role: PI.
MCCART17G0(McCarty) 11/01/17– 10/31/19
Cystic Fibrosis Foundation, Extramural grant
Title: Regulation of CFTR function by lipids and lipid-mediated signaling This proposal targets an area of CFTR biology that is relatively untouched: determining how CFTR channel activity is regulated by lipids. Our preliminary data suggest that SMase-mediated inhibition of CFTR occurs by a novel mechanism, which may alter responsiveness of the airway epithelium to modulator therapies. Role: Collaborator.
No number (Tirouvanziam) 08/01/17 – 06/31/19
Center for Cystic Fibrosis and Airways Disease Research, Intramural grant
Title: Integrative Monitoring Platform for Early Disease Events in CF (IMPEDE-CF). The goal of this project is to establish a cohort of infants with CF who will be studied using state-of-the-art imaging by computed lung tomography, and deep cellular and molecular phenotyping, in order to improve care. Role: PI.
5R01HL126603 + Suppl 01S1 and 01-S2 (Tirouvanziam) 09/14/15 – 06/30/19
NIH/NHLBI, Extramural grant
Title: Contribution of neutrophils to early airway disease in cystic fibrosis children. This project leverages a unique cohort of infants and children with CF followed longitudinally for structural lung damage to determine the role of neutrophils in airway disease progression. Role: PI.
MCCART15RC (McCarty) 07/01/15 – 06/30/19
Cystic Fibrosis Foundation, Extramural grant
Title: CF@LANTA CF Research & Development Program (RDP). The overarching goal of the RDP Center is to promote interdisciplinary research into the pathogenesis of CF and translate this new knowledge into therapeutic strategies for this life shortening disease. Role: Core Technical co-Director.
0000046269 (Tirouvanziam, Feldman) 04/01/18 – 03/31/19
Celtaxsys, Inc., Research contract
Title: Investigation of LTB4 involvement in blistering skin diseases. This study will use a combination of pathological analyses of tissues sections and high-content assays of blood and blister fluid to assess the impact of LTB4 signaling in blistering skin diseases. Role: Co-PI.
TIROUV15A0 (Tirouvanziam) (NCE) 04/01/15 – 03/31/19
Cystic Fibrosis Foundation Therapeutics, Extramural grant
Title: Impact of the resistin / insulin / IGF1 axis on CF inflammatory disease. The goals of this study are to determine the relationship between elevated resistin levels in CF airway fluid and airway disease, and explore strategies for modulating resistin / insulin / IGF1 signaling for potential therapeutic benefit. Role: PI.
W911NF-16-C-0008 (Tirouvanziam, Galinski, Gutierrez) 03/09/16– 03/08/19
DARPA, Extramural contract
Title: Host acute model of malaria to study experimental resilience (HAMMER). The major goal of this proposal is to use non-human primate model of malaria to discover novel therapeutic modalities to increase host resilience to acute infection. Role: Co-PI.
Principal investigator:
Rabindra Tirouvanziam, PhD, Engineer. Rabin earned his engineering degree in Biotechnology at the Paris Institute of Technology for Life, Food and Environmental Sciences (Agro-Paris Tech, France) in 1994 and his PhD in Developmental Biology, Lung Physiology and Immunology at the College de France and CNRS (Paris, France) in 1998. He then trained as a Postdoctoral Fellow (1999-2004), Research Associate (2004-2008) and Instructor (2008-2011) at Stanford University (CA, USA). He joined Emory University as an Assistant Professor in the Department of Pediatrics, and a training faculty in the Immunology and Molecular Pathogenesis (IMP) graduate program in 2011. >> LinkedIn
Research staff:
Milton Brown, PhD. Milton is a Research Assistant Professor in the Department of Pediatrics. He joined the lab in 2011. >> LinkedIn
Postdoctoral fellows:
Filled position - Bioinformatics. Details pending.
Filled position - Immune signaling. Details pending.
PhD students:
Bijean Ford, BSc. 2015- (IMP Class of 2014) >> LinkedIn
Brian Dobosh, BSc. 2018- (IMP Class of 2017) >> LinkedIn
Vincent Giacalone, BSc. 2018- (IMP Class of 2017) >> LinkedIn
PhD rotation students:
Details pending.
MSc students [co-mentoring with Prof. Limin Peng, Rollins School of Public Health]:
Jeffrey Chou, BSc. 2017- (MPH Class of 2019 - Biostatistics)
Sarita Mohanty, BSc. 2018- (MPH Class of 2020 - Biostatistics)
Surupa Sarkar, BSc. 2018- (MPH Class of 2020 - Biostatistics)
Niya Xiong, BSc. 2017- (MPH Class of 2020 - Biostatistics)
Undergraduate students:
Vacant position. Upon recommendation by TAs.
*See Alumnae & alumni in other tab*
Ten most recent (updated March 2019):
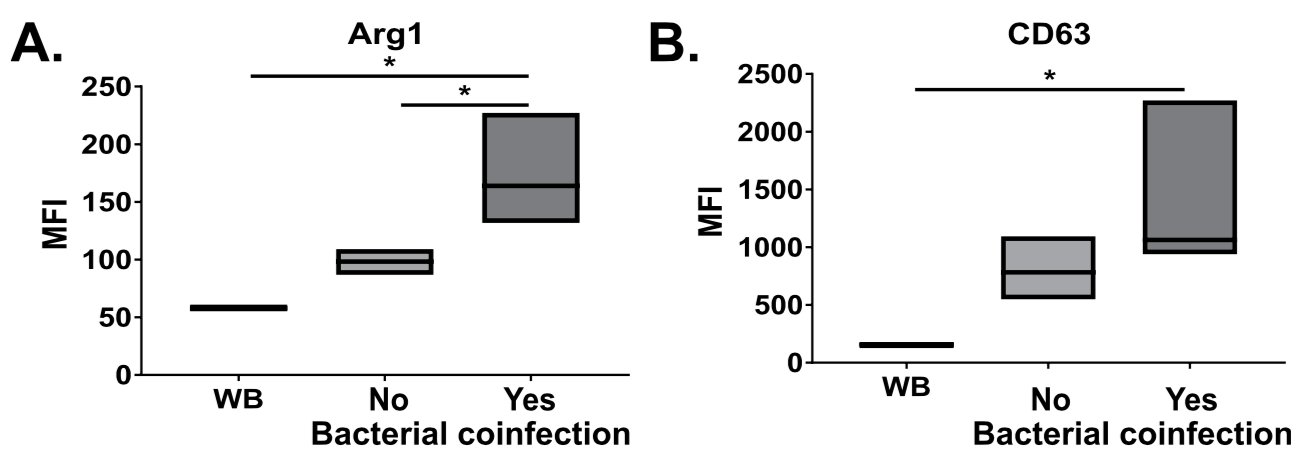
1: Grunwell JR, Giacalone VD, Stephenson S, Margaroli C, Dobosh BS, Brown MR, Fitzpatrick AM, Tirouvanziam R. Neutrophil Dysfunction in the Airways of Children with Acute Respiratory Failure Due to Lower Respiratory Tract Viral and Bacterial Coinfections. Sci Rep. 2019 Feb 27;9(1):2874. doi: 10.1038/s41598-019-39726-w. PubMed PMID: 30814584; PubMed Central PMCID: PMC6393569.
2: Tangpricha V, Lukemire J, Chen Y, Binongo JNG, Judd SE, Michalski ES, Lee MJ, Walker S, Ziegler TR, Tirouvanziam R, Zughaier SM, Chesdachai S, Hermes WA, Chmiel JF, Grossmann RE, Gaggar A, Joseph PM, Alvarez JA. Vitamin D for the Immune System in Cystic Fibrosis (DISC): a double-blind, multicenter, randomized, placebo-controlled clinical trial. Am J Clin Nutr. 2019 Mar 1;109(3):544-553. doi: 10.1093/ajcn/nqy291. PubMed PMID: 30793177.
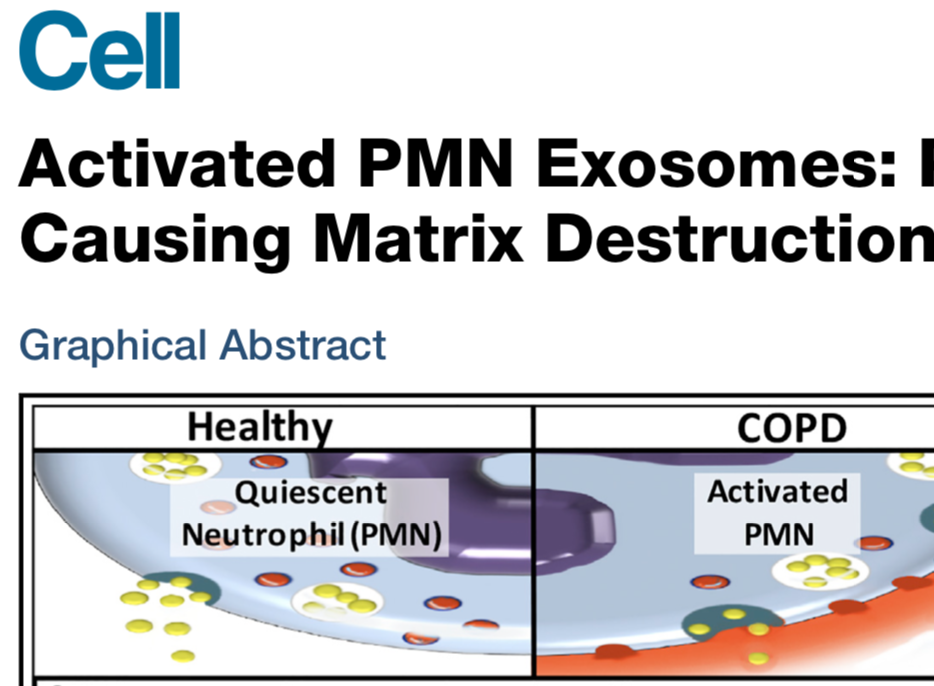
3: Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, Abdul Roda M, Xu X, Rezonzew G, Viera L, Dobosh BS, Margaroli C, Abdalla TH, King RW, McNicholas CM, Wells JM, Dransfield MT, Tirouvanziam R, Gaggar A, Blalock JE. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell. 2019 Jan 10;176(1-2):113-126.e15. doi: 10.1016/j.cell.2018.12.002. PubMed PMID: 30633902; PubMed Central PMCID: PMC6368091.
4: Margaroli C, Garratt LW, Horati H, Dittrich AS, Rosenow T, Montgomery ST, Frey DL, Brown MR, Schultz C, Guglani L, Kicic A, Peng L, Scholte BJ, Mall MA, Janssens HM, Stick SM, Tirouvanziam R; AREST-CF, and IMPEDE-CF. Elastase Exocytosis by Airway Neutrophils Associates with Early Lung Damage in Cystic Fibrosis Children. Am J Respir Crit Care Med. 2018 Oct 3. doi: 10.1164/rccm.201803-0442OC. [Epub ahead of print] PubMed PMID: 30281324.
5: Grunwell JR, Stephenson ST, Tirouvanziam R, Brown LAS, Brown MR, Fitzpatrick AM. Children with Neutrophil-Predominant Severe Asthma Have Proinflammatory Neutrophils With Enhanced Survival and Impaired Clearance. J Allergy Clin Immunol Pract. 2019 Feb;7(2):516-525.e6. doi: 10.1016/j.jaip.2018.08.024. Epub 2018 Sep 5. PubMed PMID: 30193935; PubMed Central PMCID: PMC6363859.
6: Chandler JD, Margaroli C, Horati H, Kilgore MB, Veltman M, Liu HK, Taurone AJ, Peng L, Guglani L, Uppal K, Go YM, Tiddens HAWM, Scholte BJ, Tirouvanziam R*, Jones DP*, Janssens HM*. Myeloperoxidase oxidation of methionine associates with early cystic fibrosis lung disease. Eur Respir J. 2018 Oct 10;52(4). pii: 1801118. doi: 10.1183/13993003.01118-2018. Print 2018 Oct. PubMed PMID: 30190273. Co-senior authors
7: Chandler JD, Horati H, Walker DI, Pagliano E, Tirouvanziam R, Veltman M, Scholte BJ, Janssens HM, Go YM, Jones DP. Determination of thiocyanate in exhaled breath condensate. Free Radic Biol Med. 2018 Oct;126:334-340. doi: 10.1016/j.freeradbiomed.2018.08.012. Epub 2018 Aug 22. PubMed PMID: 30144632; PubMed Central PMCID: PMC6166650.
8: Forrest OA, Chopyk DM, Gernez Y, Brown MR, Conrad CK, Moss RB, Tangpricha V, Peng L, Tirouvanziam R. Resistin is elevated in cystic fibrosis sputum and correlates negatively with lung function. J Cyst Fibros. 2019 Jan;18(1):64-70. doi: 10.1016/j.jcf.2018.05.018. Epub 2018 Jun 21. PubMed PMID: 29937317.
9: Forrest OA, Ingersoll SA, Preininger MK, Laval J, Limoli DH, Brown MR, Lee FE, Bedi B, Sadikot RT, Goldberg JB, Tangpricha V, Gaggar A, Tirouvanziam R. Frontline Science: Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J Leukoc Biol. 2018 Oct;104(4):665-675. doi: 10.1002/JLB.5HI1117-454RR. Epub 2018 May 9. PubMed PMID: 29741792.
10: Hartl D, Tirouvanziam R, Laval J, Greene CM, Habiel D, Sharma L, Yildirim AÖ, Dela Cruz CS, Hogaboam CM. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J Innate Immun. 2018;10(5-6):487-501. doi: 10.1159/000487057. Epub 2018 Feb 13. PubMed PMID: 29439264; PubMed Central PMCID: PMC6089674.
Prior publication records available on
>> Pubmed page
>> Google Scholar page
Research staff:
Marcela Preininger, BSc. 2012-13 (Technician) >> LinkedIn
Postdoctoral fellows:
Sarah Ingersoll, PhD. 2012-15 >> LinkedIn
Timothy Beaty, MD. 2012-14 (Pediatric Pulmonology Fellowship Program) >> LinkedIn
Haitham Shahrour, MD. 2016-18 (Pediatric Pulmonology Fellowship Program) >> LinkedIn
PhD students:
Julie Laval, PhD. 2011-14. >> LinkedIn
Osric Forrest, PhD. 2012-18. >> LinkedIn
Camilla Margaroli, PhD, MSc. 2016-19. >> LinkedIn
MSc students [co-mentoring with Prof. Limin Peng, Rollins School of Public Health]:
Ishaan Dave, MSc. 2015-17 (MPH Class of 2017 - Biostatistics)
Alexandria Portelli, MSc. 2016-18 (MPH Class of 2018 - Biostatistics)
Alexander Taurone, BSc. 2017-18 (MPH Class of 2019 - Biostatistics)
Undergraduate students:
Sanjana Rao. 2014-16 (Emory, Class of 2016, Honors thesis)
Yash Patel. 2015-17 (Emory, Class of 2017, Honors thesis)
Helen Yiwen Li. 2015-17 (Emory, Class of 2019)
Parth Mody. 2017-18 (Emory, Class of 2021)
Sonya Vijayvargiya. 2017-18 (Emory, Class of 2021)
Adi Swaminathan. 2017-18 (Emory, Class of 2021)
*See Current group members in other tab*